Ex vivo model in Oncology: The Oncogramme
Despite advances in both in vitro human cell-based models and in vivo animal models, 97% of oncology drugs that enter clinical trials fail to receive regulatory approval. To overcome this poor approval rate, we need clinically patient-relevant translational systems that better mimic the heterogeneity and complexity of human tumors to:
- Understand drug effects on the tumor cells
- Gain more accurate insights beyond general cell viability
- Evaluate new drugs
- To be able to accompany the new treatment in the clinical phase as a complementary diagnosis
The Oncogramme ex vivo platform is a highly translatable functional assay for evaluating both monotherapy and combination of therapies in patient tumors cells in a standardized environment with proven clinical validity.
Advancement Over Traditional Models
Challenges associated with traditional models include:
- In vitro assays, such as 2D cell lines, lack the complexity and heterogeneity of patient primary cancer cells.
- In vivo models, such as PDX, are more complex to develop and have lower throughput.
The Oncogramme platform provides an alternative approach by keeping the complexity of the tumor (primary culture of patient cancer cells) and including a standardized production process with established clinical predictivity.
A unique, standardized approach
|
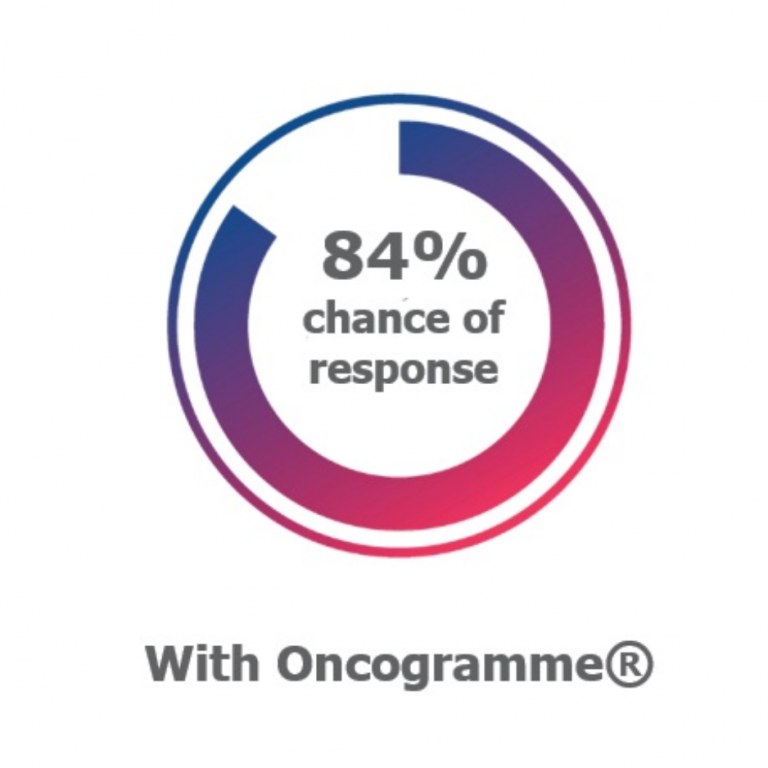
In a few words, the Oncogramme® is a standardized functional assay that enables the quantitative evaluation of the anti-cancer capacities of a therapeutic molecule or combination of molecules, directly on cancer primary cells extracted from a patient's tumor. As the results of this in vitro evaluation are more than 80% correlated with the results obtained in patients, this functional assay makes it possible to secure your results with a test that is as close as possible to the patient, upstream of the clinical phase (R&D, preclinical phase) or during clinical studies (complementary diagnosis). A drug, or combination of rugs, identified by the Oncogramme® as having an anti-cancer effect on a patient's cancer cells will have a real effect on the patient's tumor during treatment in over 80% of cases. |
 |
|
| The high predictivity of the Oncogramme is based on 3 proprietaries technology pillars: | ||
| 1. Multi steps standardized procedure with clinical data correlations for validation | 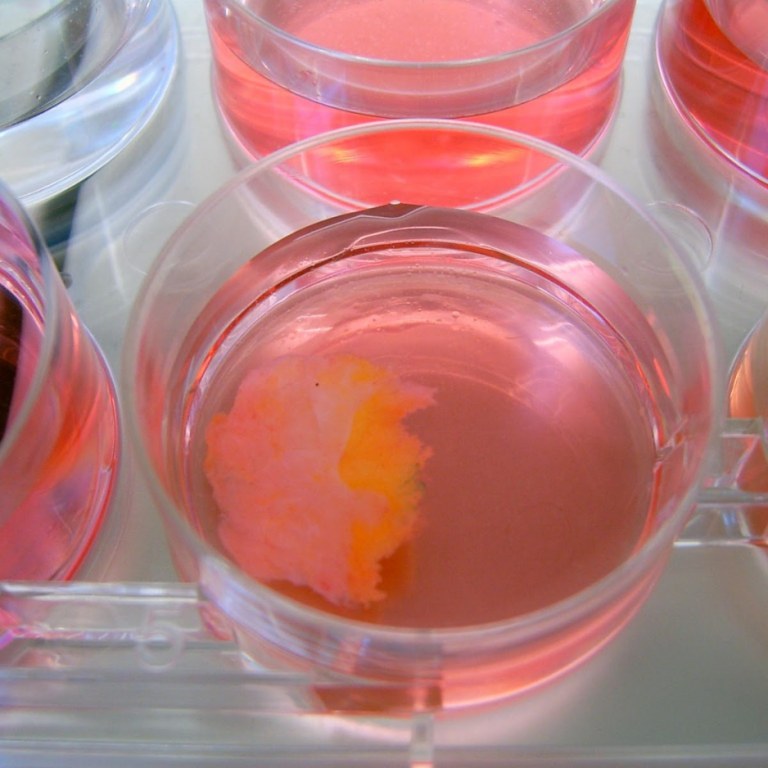 |
|
|
2. Complex proprietary kit of culture media and reagents, the OncoMiD-Via® Series, for sample shipping/preparation and tumor culture of all cancer cells for an optimal analysis. OncoMiD® medium and reagents provide a chemically defined solution for the transport, separation, and culture of tumour cells. These chemically defined and validated media support the transport and dissociation of tumor samples, enabling subsequent ex vivo culture |
 |
|
|
Types of tumors treated :
|
 |
|
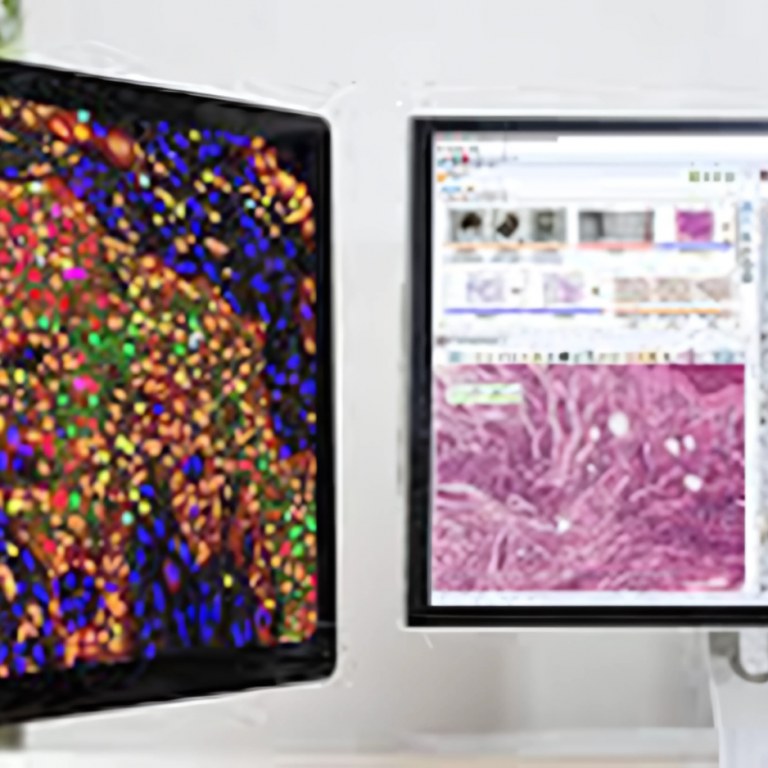
3. Unique proprietary algorithm for automated counting correlated with clinical data. |
 |
The Oncogramme is currently validated for preclinical and clinical studies in colorectal, breast, ovarian and lung cancers, as well as glioblastoma, and is ready for development in other areas.
Example of correlation between a predicitive biomarker and tumor cell effective response : HER2 Breast tumor and Herceptin
A diagnostic version (CE-IVD) for metastatic colorectal cancer is already on the market.
Comparison of Models
Poor (x), Medium (~), High (V)
|
Attributes |
Oncogramme |
Cell lines 2d culture |
Organoids |
in vivo |
|
Physiological complexity |
V |
X |
~ |
V |
|
Throughput |
V |
V |
V |
X |
|
Translatability to the clinic |
V |
x |
~ |
~ |
In R&D or preclinical, the Oncogramme® allows:
- To test the efficacy of a new molecule on tumor cells from patients, by a standardized and correlated with clinical data test, and thus identify new biomarkers.
- To compare the efficacy of a drug candidate versus other therapies, whether or not present on the market.
- To study the potential synergy effects with other molecules, present or not on the market. This ability makes it possible, in particular in the case of good synergistic effects, for example with immunotherapies, to bring a drug candidate, which alone would be in 2nd or 3rd place in terms of efficacy, to first place.
By including the Oncogramme® in your clinical studies or after a clinical study, you can:
- Secure your clinical studies by targeting the right patients using a functional assay with more than 80% of predictivity
- Obtain a companion or complementary test to promote your molecule.
- Repositioning a treatment
Our Oncogramme platform is available to help you secure your preclinical and clinical oncology experiments.
Contact our technical support team to explore how we help you.
