Biomarker Development and Validation Services
Biomarkers are important when it comes to the development of novel drugs and mechanisms and are also gaining importance as companion diagnostic markers.
At CliniSciences, we are dedicated to providing innovative solutions and products that support the development of new therapeutics. Our CRO team is committed to utilizing cutting-edge technologies and ensuring the accuracy and reliability of the results to meet your exploratory biomarker discovery needs.
The importance of Biomarkers in drug discovery and development is constantly growing. In drug development, Biomarkers are used to confirm mechanism of action, monitor disease progress and assess drug efficacy and safety.
In addition, Biomarkers are deployed for pharmacokinetic / pharmacodynamic (PK / PD) modeling. Because of the central role throughout all phases of drug development, Biomarker assessment requires robust and sensitive assays with high accuracy and precision. CliniSciences has longstanding experience in Biomarker assessment, including assay development and validation.
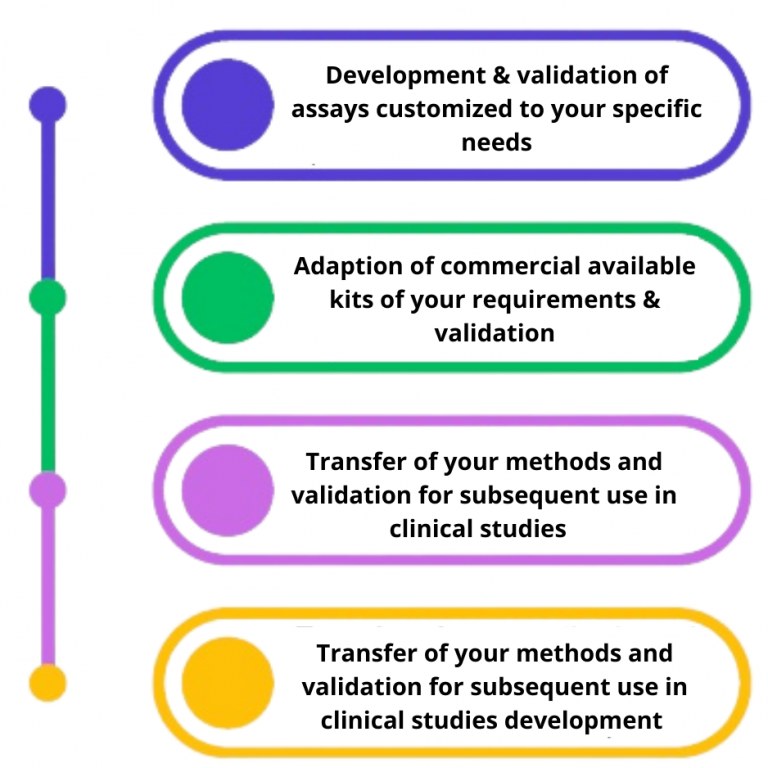
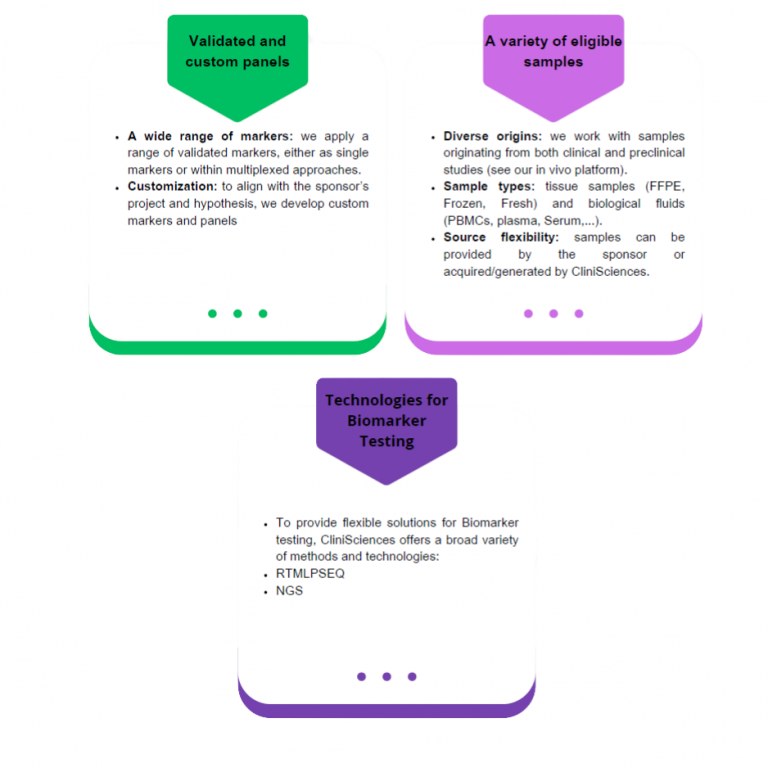
The choice of the analytical technology and method can be critical for successful Biomarker testing. CliniSciences gives emphasis on scientific discussion to identify your needs and aims. We provide flexibility for:

Oncotarget NGS Panel for biomarker detection
The ONCOTARGET Genomic Profiling Panel, a cutting-edge solution designed to revolutionize cancer biomarker detection. ONCOTARGET is a complete NGS panel that we can tailor to your biomarker detection needs.
 |

RT-MLPSEQ: A new technology to characterize cancers Gene expression and fusion transcripts
The RT-MLPSeq custom solution from CliniSciences and GeneXpath allows you to assess the mRNA expression level or the presence of fusion transcripts of 10 to 100 markers of interest of your choice.
 |

